Metallosis of the Resurfaced Hip
by James W. Pritchett MD (added January 24, 2011)
Introduction
Metallosis of the hip is usually defined as aseptic fibrosis, local necrosis, or loosening of the prosthesis secondary to metallic corrosion and release of wear debris.8 It has also been defined as gray discoloration of the tissues of the joint, pain, an effusion, and elevated serum metal levels. Metallosis has been found with stainless steel, titanium, and cobalt chromium alloy femoral prostheses articulating either with a similar metal or (rarely) with a polymer acetabular component. Titanium and stainless steel femoral head prostheses are no longer used, so today metallosis usually refers to tissue changes observed following the use of cobalt chromium-on cobalt chromium (metal-on-metal) implants. Metal-on-metal hip prostheses have been in common use for total hip replacement and almost all current hip resurfacing prostheses are metal-on-metal.12
Cobalt is in the middle of the periodic table and is called a transition metal. Cobalt’s atomic number is 27 and its molecular weight is 58.9. Transition metals have many uses and are valued for their strength. Cobalt is found in vitamin B12 and is essential for oxygen transport32. Cobalt sulfate has been used as a pigment in porcelains and glass since at least 2600 BC. It is also much in demand for use in rechargeable batteries, and as an additive to soil and animal feed. Cobalt sulfate was used to improve the stability of foam in beer and to treat some forms of anemia not responsive to other treatment.32,34 Cobalt chloride is also commonly used commercially to produce a blue color. It is used in Europe in cements and detergents. There is more sensitivity to cobalt in Europe than in the United States. Cobalt occasionally produces dermatitis but there is less hypersensitivity to cobalt than to other metals, such as stainless steel and other nickel-containing alloys.9
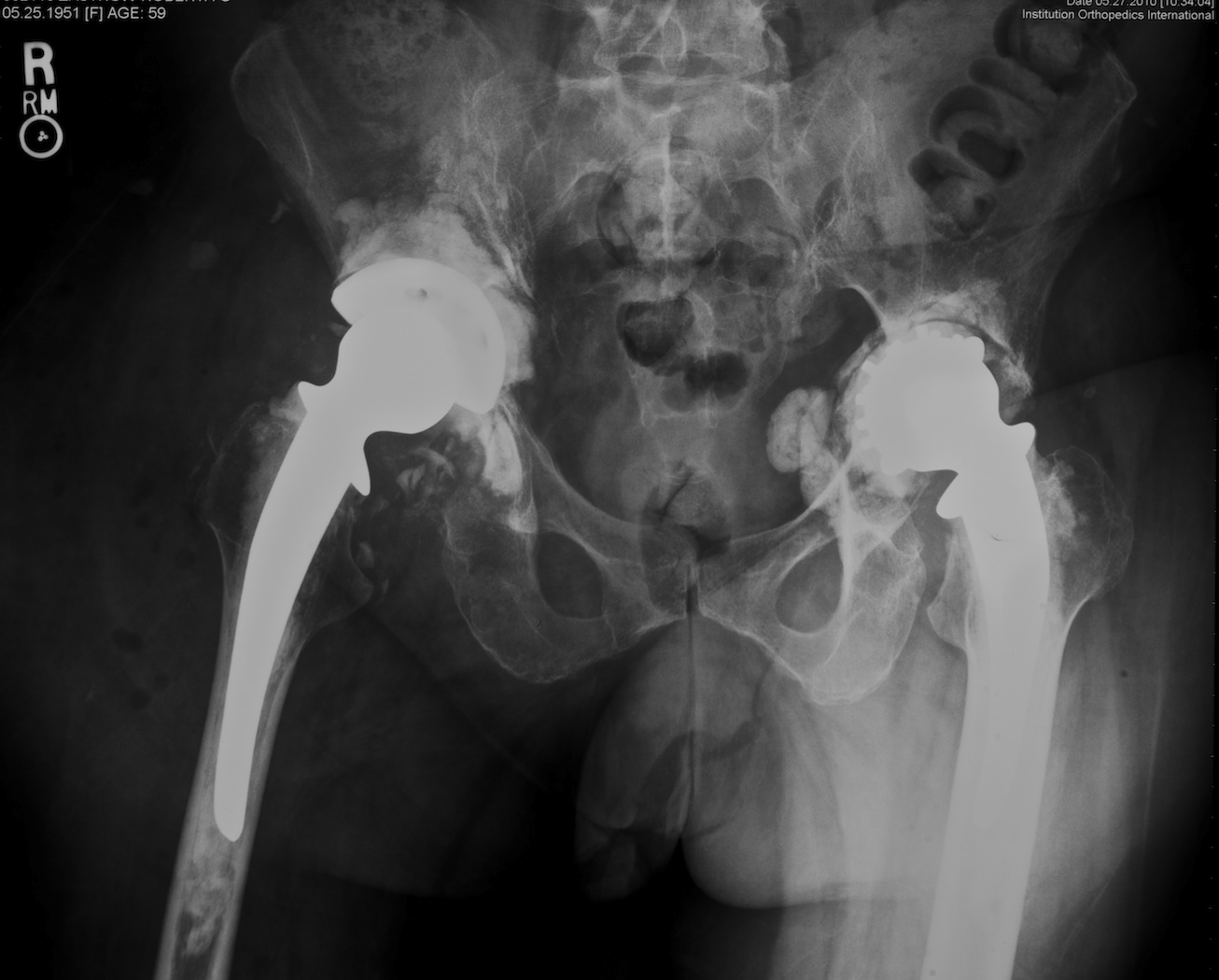
Fig. 1. This anteroposterior pelvis radiograph shows a 58 year-old woman 40 years after receiving bilateral McKee-Farrar total hip replacements. On the left the original metal-on-metal prosthesis demonstrates acetabular loosening. On the right a revision using a custom made two-piece titanium and polyethylene acetabular prosthesis has been performed.
Cobalt Chromium Hip Prostheses
A cobalt chromium hip prosthesis was first used in 1938 for cup arthroplasty by Dr. Marion Smith-Peterson.33 The original alloy (Vitallium) came from the dental industry, where it was used for bridges, dentures, and orthodontia. Dr. Smith-Peterson’s dentist, Dr. J.W. Cook, called his attention to the work of Drs. CharlesVenable and Walter Stuck in 1938.33,38 H.R. Bohlman first used a Vitallium femoral head and neck replacement in 1939 by attaching a Vitallium ball to a tri-flanged nail.3 Bohlman and Austin Moore performed the first successful femoral head prosthesis implantation in 1940.21
Fig. 2. This syringe shows joint fluid that has been aspirated from a patient with metallosis 2 years following hip resurfacing surgery.
Vitallium was attractive because of its corrosion resistance and electrolytic inertness. Bohlman buried different metallic prostheses on his farm and implanted them in animals before determining they were suitably inert.3,21 All implants will ionize after implantation but cobalt chromium remains the most corrosion resistant.8,21,38
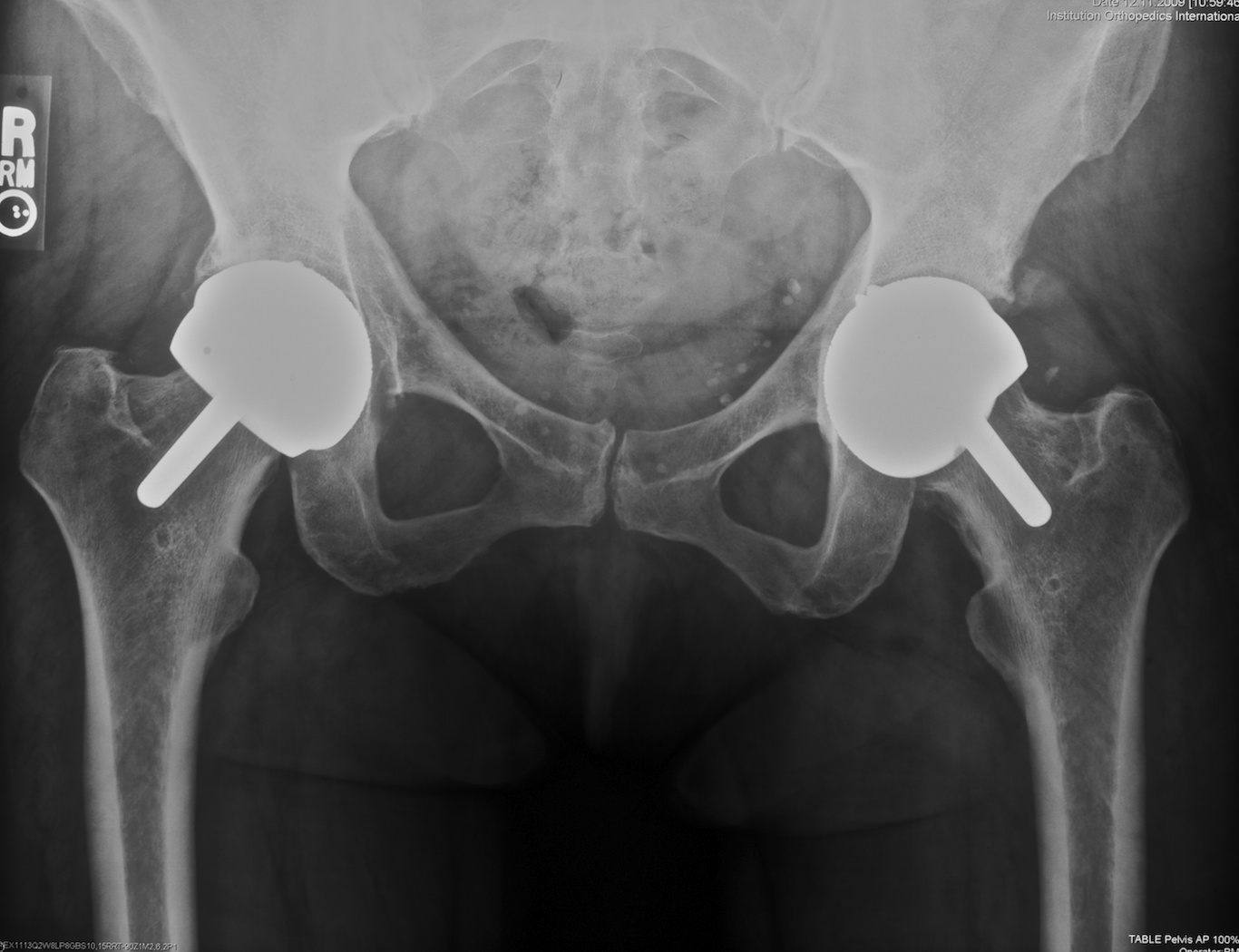
Fig. 3. This anteroposterior radiograph shows a 46 year-old woman who has undergone bilateral hip resurfacing surgery using Birmingham prostheses. On the right the outcome is successful but on the left the hip is painful from failure of osteointegration and there is a lucent line around the acetbular prosthesis.
In 1951, George McKee began using stainless steel for total hip replacement but all prostheses failed. He began using a cobalt-chromium Thompson prosthesis in 1956 and refined his McKee-Farrar Prosthesis in 1966 (Fig. 1).19,20,35 Dr. Charles O. Townley originally used stainless steel for femoral head resurfacing in 1951 but also quickly moved to cobalt-chromium.36 The Peter Ring prosthesis, starting in 1964, also utilized cobalt chromium.31 Marshall Urist, Earl McBride, and Maurice Mueller all used cobalt-chromium but Sir John Charnley used stainless steel, articulating it with polyethylene after a failed attempt using Teflon.4,18,22,37
McKee-Farrar Arthroplasty
McKee in 1971 first reported metallosis following metal-on-metal total hip replacement. He described two patients who developed pain 3½ and 4½ years following total hip replacement. Both had sterile necrotic material at exploration.20 Seven additional patients were described by Jones, et al in 1975. Their patients developed symptoms between 9 months and 4 years following McKee-Farrar total hip replacement.13
Affected patients developed progressive pain and a feeling of instability. Bone loss with soft tissue necrosis was found in all cases. Two patients had spontaneous dislocations. The tissues were stained green or grey and a paste was found around a thickened capsule. Highly elevated levels of cobalt were found in the serum and joint fluid. Large joint effusions with either rust, green, cloudy yellow, or grey-colored fluid were found in all cases.13,20
McKee-Farrar prostheses were manufactured as a matched set with the acetabular component made, matched, and tested to accompany the femoral prosthesis.19 This manufacturing technique would be desirable today but no manufacturer offers their implants prepared and tested together. McKee-Farrar hip replacement was abandoned in the early 1970s as other prostheses proved more successful.
Modern Metal-on-Metal Resurfacing
Metal-on-metal hip resurfacing was performed initially by Dr. Edward Haboush in 1951 and occasionally until the mid 1970’s.10,22,26,28 There was no mention of metallosis in the early reports. With superior metallurgy, metal-on-metal resurfacing began again in 1988. It increased in popularity and in 2006 a full FDA approval for metal-on-metal resurfacing was obtained.7,12 All implant manufacturers indicated their metallurgy had improved and the critical nature of component positioning was not emphasized. In fact, the large diameter of metal prostheses was felt to protect against dislocation even in instances of component malposition. Starting in 2008 reports of “pseudotumours” with metal-on-metal resurfacing began appearing in the literature.25 Such cases are now regularly reported. There is conflicting information about the incidence and predisposing factors. Implant manufacturers and some surgeons report that women and/or smaller size patients, with or without steep abduction angles, are more likely to develop metallosis.6,15
The position of the components does not by itself provide a full explanation for the development of metallosis. There are patients who develop metallosis with ideally positioned components and also, some who do not develop metallosis with poor component position. The Mayo Clinic found no relationship between metallosis and component positioning.2 The reports of metallosis with the McKee-Farrar prostheses also do not associate component position with metallosis. Radiographs from the several reports available do not support the conclusion that vertical component positioning was a problem with early metal-on-metal prostheses.19,20,31,40-42
Diagnosis of Metallosis
The symptoms of metallosis include pain, a sense of instability, and increasing noise coming from the hip. The symptoms evolve over several months and are always progressive. Metallosis has not been proven earlier than 9 months postoperatively but symptoms always present within the first 4 years following surgery. Pain that remains or appears immediately after recovery interval following surgery is not due to metallosis. Up to 18% of hip resurfacing patients experience groin pain following surgery but only 2% to 5% has metallosis.1
Several other causes for symptoms following metal-on-metal hip arthroplasty require consideration. These include implant loosening, periprosthetic fracture, osteonecrosis, infection, tendinitis, impingement, and referred pain. Selective injections and advanced imaging can be very helpful in discovering the cause of symptoms.
Cobalt Levels
Serum cobalt levels are useful in predicting metallosis. However, all patients have elevated cobalt levels following metal-on-metal joint replacement. Thus, the cobalt level should be measured several months or a year following surgery to avoid a misleading result caused by the wearing-in process of the prosthesis.11,15 For unilateral metal-on-metal joint resurfacing, a cobalt level ≤ 4 µg/L is expected and for bilateral resurfacing the cobalt level is generally < 9 µg/L. Cobalt levels > 100 µg/L are found occasionally. Patients with equivocal levels should be followed over time with repeated testing. If the cobalt level is increasing, metallosis should be suspected. In some laboratories, testing of the joint fluid is possible but usually the appearance of the aspirated fluid is information enough to diagnosis metallosis (Fig 2).
Noise
All artificial joints make noise. With hard-on-polyethylene joints, clicking is most often reported. Acoustical analysis of noise from metal-on-metal and ceramic-on-ceramic prostheses demonstrates that every implant produces significant noise. Most often, the natural frequency of the noise generated is above the human audible range. Thinner acetabular shells produce lower frequency noise. When two very thin shells are used, such as with resurfacing, noise within the audible range is possible. Resurfaced hips with metallosis present reliably produce an audible or palpable sense of noise or vibration.
Audible sounds from ceramic-on-ceramic hip prostheses have received considerable attention since 2005. Squeaking occurs in up to 10% of hips with ceramic-on-ceramic prostheses but rarely presents a clinical issue.30,43 Squeaking also occurs with metal-on-metal hip prostheses in up to 10% of patients. It is self-limited and is rarely of clinical concern. A clunking sound is much more common with metal-on-metal prostheses and occurs in up to 28% of patients. Clunking is more prevalent in the first several months following surgery but may continue.
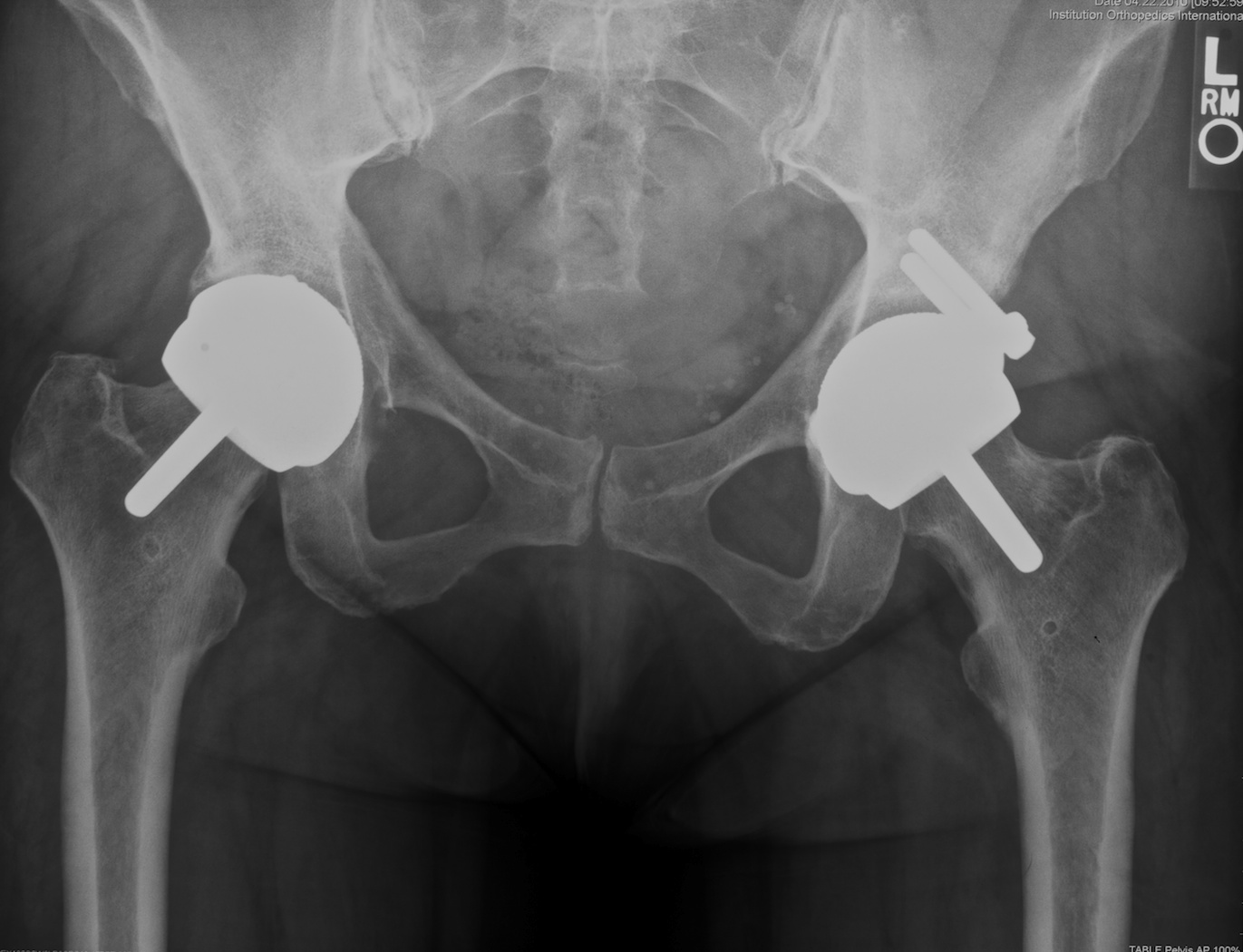
Fig. 4. A revision of the left acetabular component has been performed using a dysplasia component with supplemental screw fixation.
The sounds coming from a hard-on-hard bearing prosthesis are produced by forced vibration. When loss of fluid film lubrication occurs, with hard bearings, high levels of friction may result. Loss of fluid film lubrication may be caused by edge loading, impingement, third-body particles, bearing surface damage, or alteration in the joint fluid. Edge loading increases the coefficient of friction several fold.40-42 Aspiration of the hip joint revealed ceramic particles in all patients with squeaking hips in one study of ceramic-on-ceramic hip prostheses.30 Joint aspiration of metal-on-metal hips that have become progressively noisier shows evidence of metal staining in most every instance. The key issue with noise production from a metal-on-metal hip is its pattern over time. If the noise lessens or remains stable, the outcome is likely favorable. Noise that becomes more prominent is suggestive of metallosis. Clunking rather than squeaking is the important noise for a metal-on-metal hip.
In the absence of metallosis squeaking and other noise from joint implants generally follows a benign course. We have had success in injecting squeaking hip joints with hyaluronic acid. With well functioning, well positioned components two or three injections has generally been successful in significantly reducing or eliminating squeaking.
Solubility of Cobalt
All patients with metal-on-metal hip prosthesis have elevated levels of cobalt in their hair, blood, urine, placenta, and vital organs.5 Hypersensitivity from cobalt can occur but it is relatively rare and there is no validated test to establish the diagnosis prior to implantation of a cobalt prosthesis.9 True hypersensitivity is very rare but most patients with metal-on-metal prostheses will react to skin tests for cobalt sensitivity. However, when the cobalt prosthesis is removed, patients no longer react, suggesting they are likely not hypersensitive. Therefore, there is no validated way to determine hypersensitivity to cobalt following implantation of a cobalt-containing joint prosthesis.
A metal-on-metal prosthesis presents a large surface area to surrounding body fluids. Solid metals must achieve equilibrium with the surrounding body fluids to avoid a local accrual of metal ions to a toxic level in the joint. As the cobalt is generated it must be absorbed by the lymphatics and synovial tissues. It is then circulated and excreted through the urine. In our laboratory testing, the solubility of cobalt in joint fluid varied by a factor of 4 between patients. Also, the ability to excrete cobalt varies with renal function. Patients with impaired renal function are not candidates for metal-on-metal joint prostheses.
Tissue Reaction to Elevated Cobalt Levels
Relatively advanced metallosis can present with “pseudotumors.”13 This description is appropriate as the tissues observed have a villous character reminiscent of bowel mucosa. Pseudotumors are a benign inflammatory granulomatous reaction to cobalt absorption. These masses are associated with large fluid collections and tissue necrosis is evident on histological analysis. The local inflammatory reaction should always be distinguished from infection. Markers such as C-reactive protein are not elevated and cultures are negative.
Pseudotumors regress when the local cobalt level is reduced, either by removal or revision of the prosthesis; thus, there is no need to aggressively resect the involved tissues. Osteolysis and further tissue necrosis, however, will follow if treatment is not provided. There is no nonoperative treatment for pseudotumors or metallosis.
In some patients the tissue reaction is tissue necrosis and fibrosis. Unlike with pseudotumor formation the tissues become avascular and necrotic. Adjacent to the necrotic tissue is often much thickened fibrotic soft tissues. The underlying bone is avascular with dead appearing surface.13,20
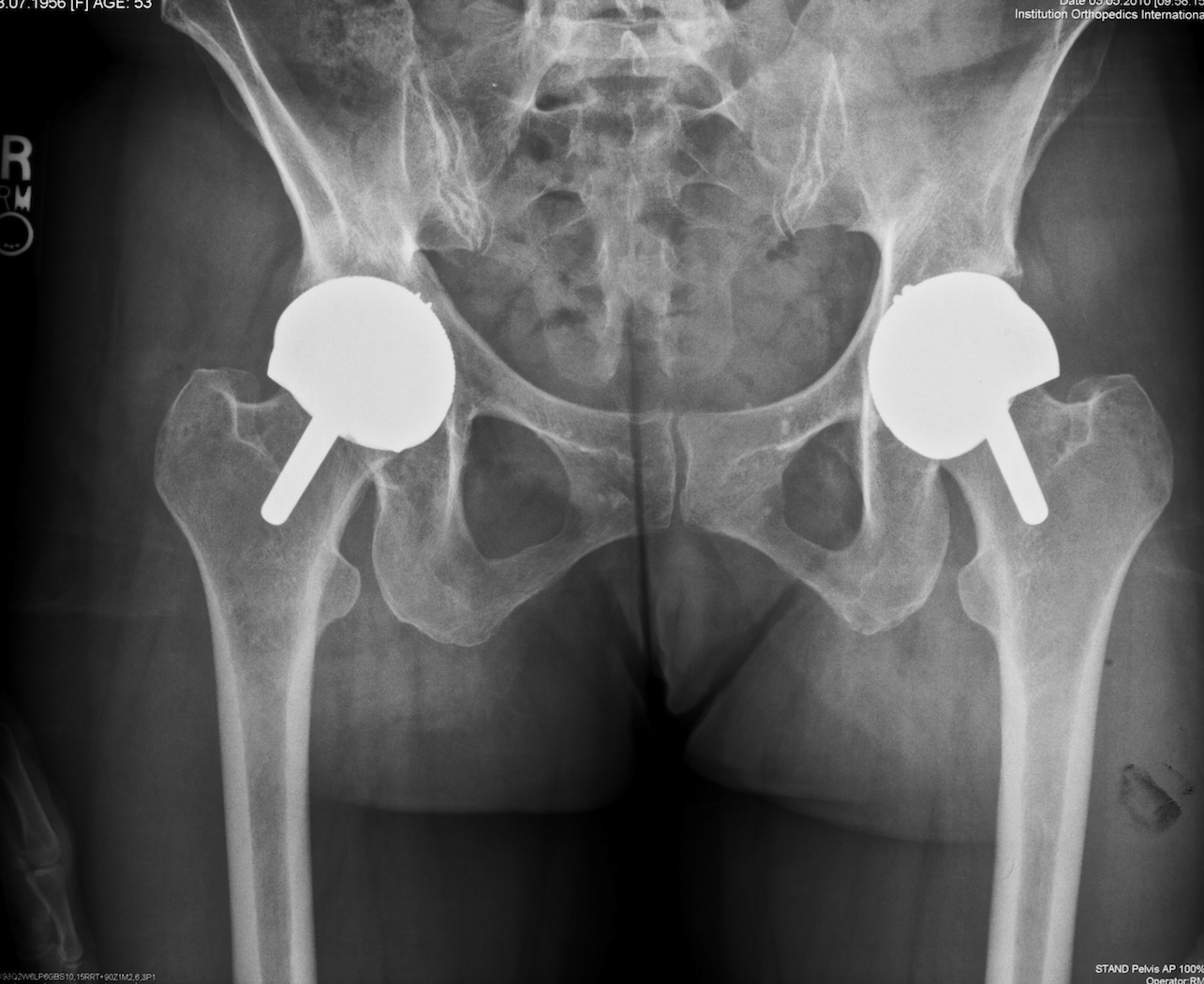
Fig. 5. This anteroposterior radiograph shows a 45 year-old women who underwent bilateral hip resurfacing surgery for dysplasia. On the right the outcome was successful but on the left she developed metallosis and the acetabular component has a vertical orientation.
Rarely patients present with little pain but severe soft tissue necrosis and/or osteolysis. The presenting symptom can be a spontaneous dislocation as the soft tissues are lost or a periprosthetic fracture. The first literature reports describe several such cases. This presentation represents the most significant challenge for later reconstruction.2,13,30
Sometimes patients have more than one type of tissue reaction to the elevated cobalt level or the primary tissue response evolves from one type to another over time. It is unknown why some patients respond differently than others.
Excluding other Diagnoses
Most patients with symptoms following metal-on-metal hip joint surgery do not have metallosis. Moreover, most patients who are given the diagnosis, even after revision surgery, do not have metallosis. Metal staining of the tissues, without noise and a very elevated cobalt level, does not warrant the serious diagnosis of metallosis. Most surgeons do not have experience with resurfacing and some do not believe there is a place for resurfacing procedures.14 Careful laboratory analysis shows many suspected cases of metallosis do not have the condition. The most common alternative diagnosis is failure of osteointegration of the acetabular component (Figs. 3 and 4). It is more difficult to osteointegrate cobalt prosthesis compared to titanium. Also, the large inner bearing diameter places high force on the implant and secondarily on the prosthesis-bone-interface. The one-piece cobalt prostheses used for resurfacing cannot accept supplemental screw fixation, further compromising the security of acetabular component fixation.
Acetabular components that have not osteointegrated can be a source of pain but they are often difficult to detect radiographically, at least initially, before radiolucent areas appear. The initial press fit limits the amount of initial pain. Within a few months, pain from poor osteointegration may be experienced. The onset of this pain is generally earlier time than the pain from metallosis. During joint aspiration, local anesthetic that is instilled into the joint can be helpful in establishing the diagnosis. Revision surgery is very successful for patients with failure of osteointegration.
Periprosthetic fractures, particularly of the femoral neck, can occur intra- or postoperatively. These may be difficult to see with radiographs and require bone or CT scans to detect. Some of these fractures will heal with time but many require revision surgery.
Infection, tendinitis, and heterotopic ossification usually can be discovered by examination and are quite treatable. Tendinitis is particularly common with resurfacing, as the joint is re-spaced during surgery, resulting in modest tendon lengthening. Most patients notice limb lengthening. 6 to 7 months following surgery most patients no longer perceive that their operative leg is longer.
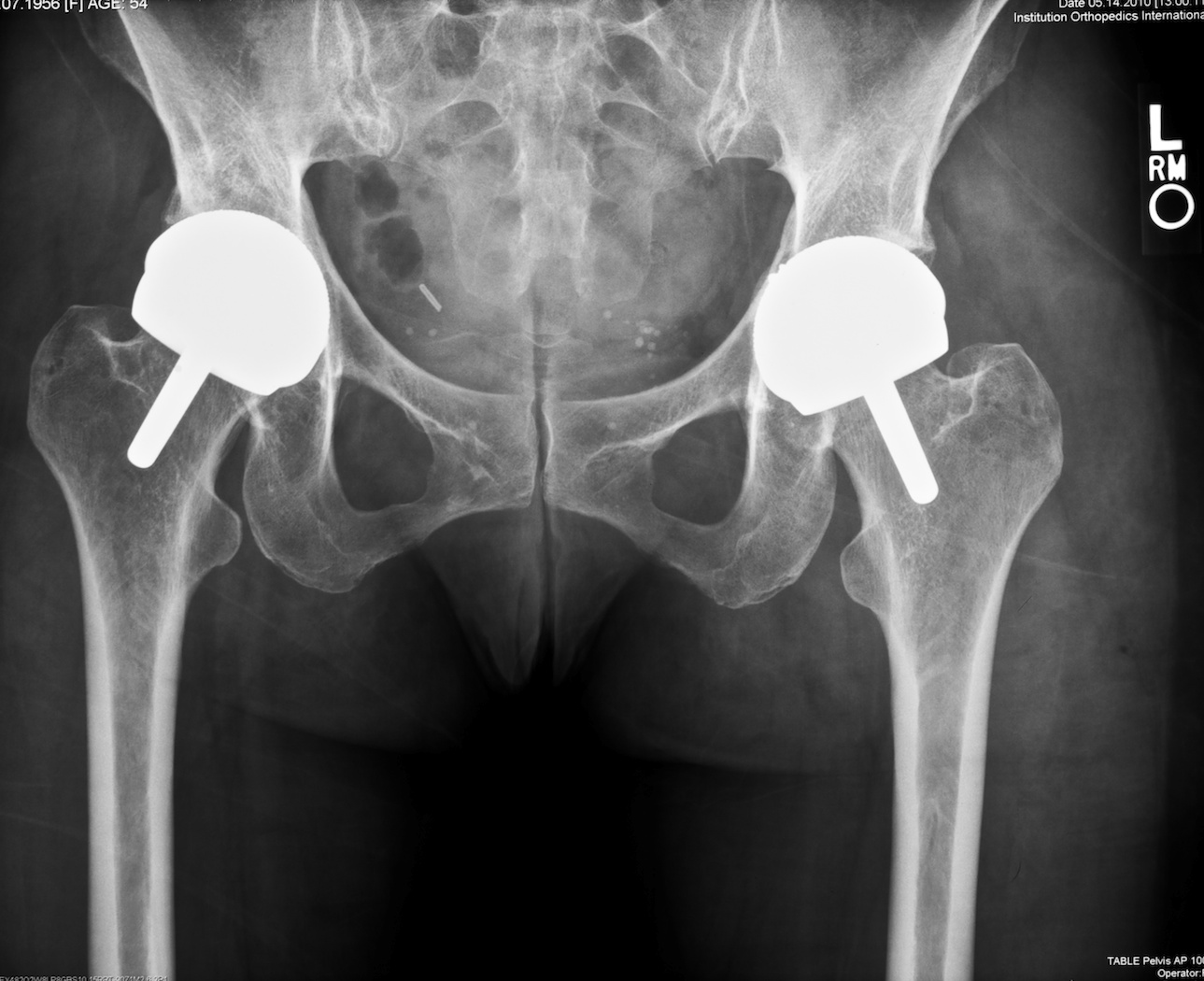
Fig. 6. A revision of the left acetabular component to a more horizontal position has been performed and the outcome is successful.
Synovitis is common after joint implant surgery. Synovioctyes are capable of proliferative overgrowth and significant effusions are common even in well performing joint replacements. Great caution is necessary in interpreting the presence of effusions on MRI or CT scans following joint resurfacing or replacement. Metaplasia of synovium to fibrous tissue that completely covers the patellar component of a knee replacement is commonly seen. Synoviocyte replication varied by a factor of ten in patients we have tested.
Treatment of Metallosis
There is no medical, physical, or nonoperative treatment for metallosis. It is not possible to chelate the excess cobalt from either the joint or serum. Once metallosis occurs, the tissue response continues and, thus, surgery is always necessary. Almost all patients, however, respond favorably to surgery. The surgical options must be tailored to the needs and desires of the patient rather than the surgeon.
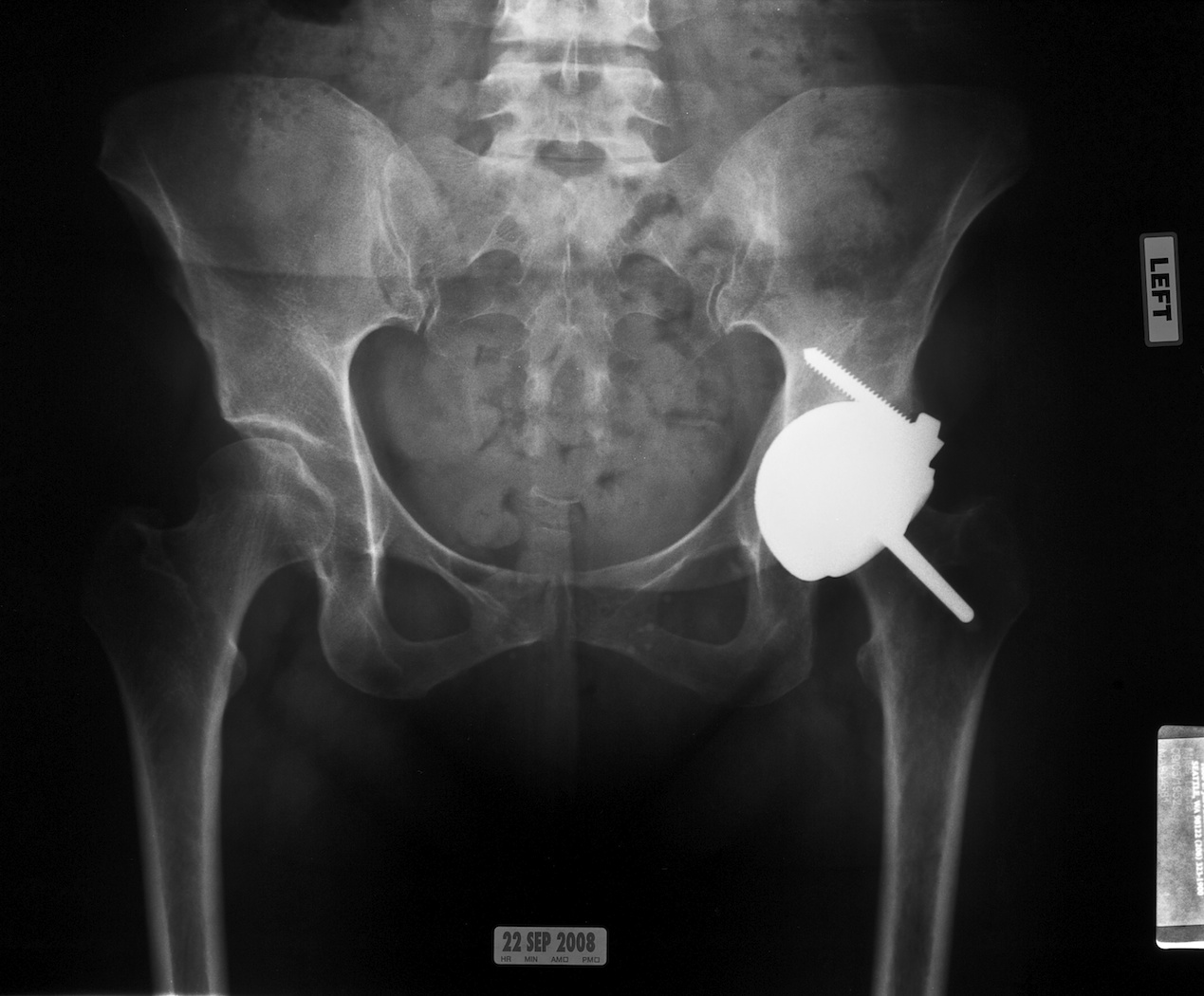
Fig. 7. This anteroposterior radiograph shows the Conserve Plus dysplasia prosthesis that has been successfully used for revision surgery in this 44 year-old woman.
Few surgeons are experienced in evaluating and treating patients with metallosis. Even fewer have the necessary extensive experience with resurfacing. If the initial outcome of the resurfacing procedure is favorable and the patient is young and active, the indications remain for hip resurfacing.
Metal-on-Metal Hip Resurfacing Revision
In most patients, the hip resurfacing can be revised successfully, usually with an acetabular-only revision.6 Most revision procedures are performed with primary resurfacing components using the same component size (Figs. 5 and 6). Outside diameters 2 - 4 mm larger are also available, if necessary, to achieve a secure press fit. Peripheral screws also can be beneficial if additional fixation is necessary. The components provided for dysplasia patients are used for this purpose (Fig. 7). Overall, revision procedures are easier than the initial surgery for patients to go through. The recovery interval is less than half that experienced with the index procedure. For an experienced revision resurfacing surgeon the procedure is readily accomplished. The original position for the resurfacing is avoided and a better position for the new acteabular component is more apparent. Often it is possible to see where the original component did not match the prepared bone. The original component migrated either at the time of impaction or later. Unlike total hip replacement, the results of revision resurfacing do not show any reduction in function or increase in complications.
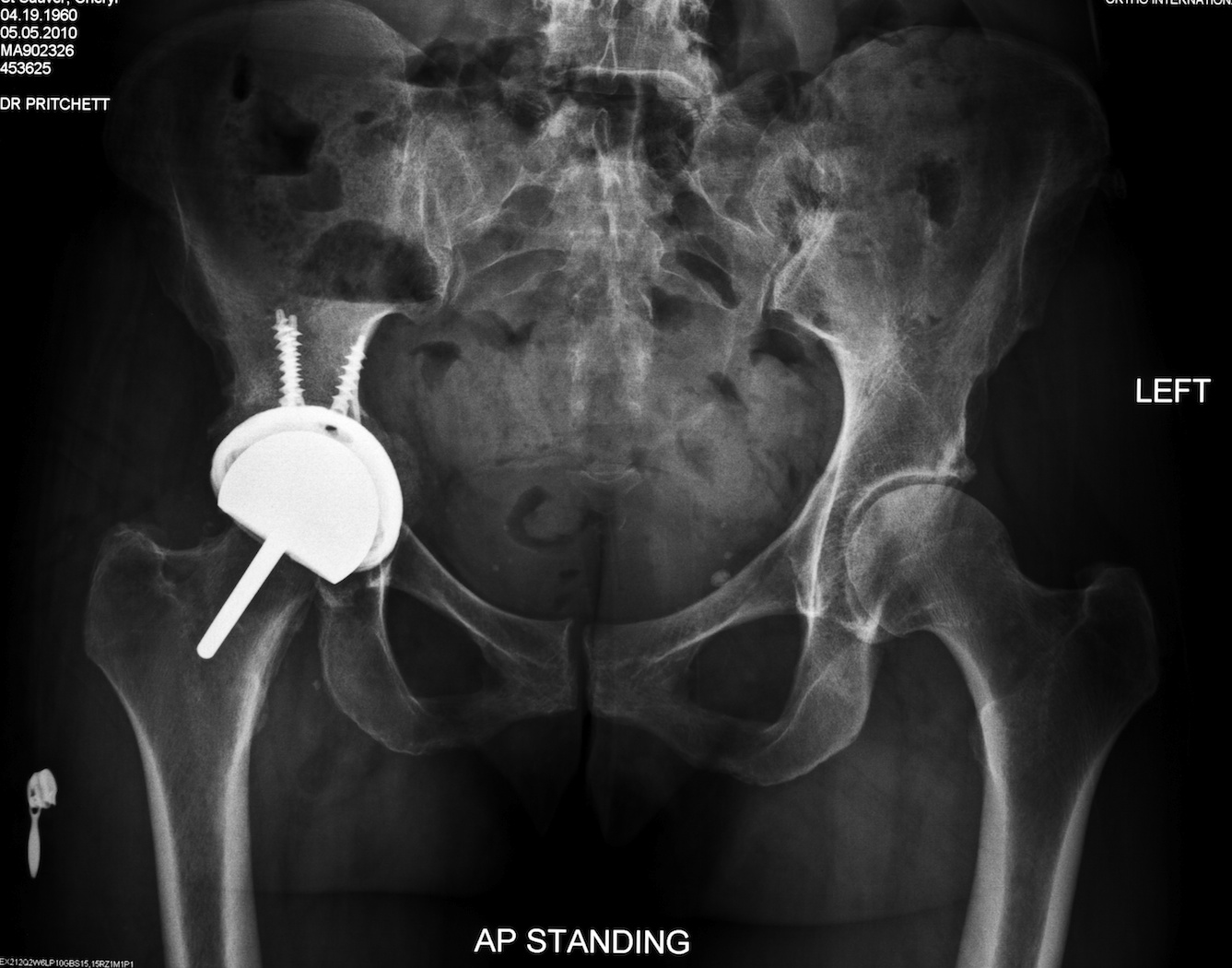
Fig. 8. This anteroposterior radiograph shows a 51 year-old woman who underwent an acetabular revision of her resurfacing prosthesis using a two-piece titanium and polyethylene prosthesis.
In recent reports, up to 40% of resurfacing acetabular prostheses are not ideally positioned even in the hands of experienced hip surgeons.23 Most of these patients are ideal candidates for revision resurfacing. The number of total hip acetabular components that are placed outside suggested parameters is 13% to 18%.16
Reasons for Acetabular Component Positioning Challenges
It is much more difficult to accurately place and secure an acetabular component during resurfacing surgery compared to total hip replacement. The access to the acetabulum is more difficult with the retained femoral head and neck in the way. Most patients presenting for resurfacing have an abnormally shaped, dysplastic native acetabulum. The presenting acetabulum is vertically oriented and attempts to preserve the bone during preparation tend to lead to increased anteversion and vertical orientation. It is very unusual to see an acetabular component positioned more vertical than the native acetabulum. Also, the acetabular bone is hard and sclerotic in the areas where there has been uneven weight bearing. Resurfacing acetabular components are driven into the acetabular bone and the harder bone in some areas and softer in other tends to tilt the acetabular component at the time of impaction or with later load bearing.
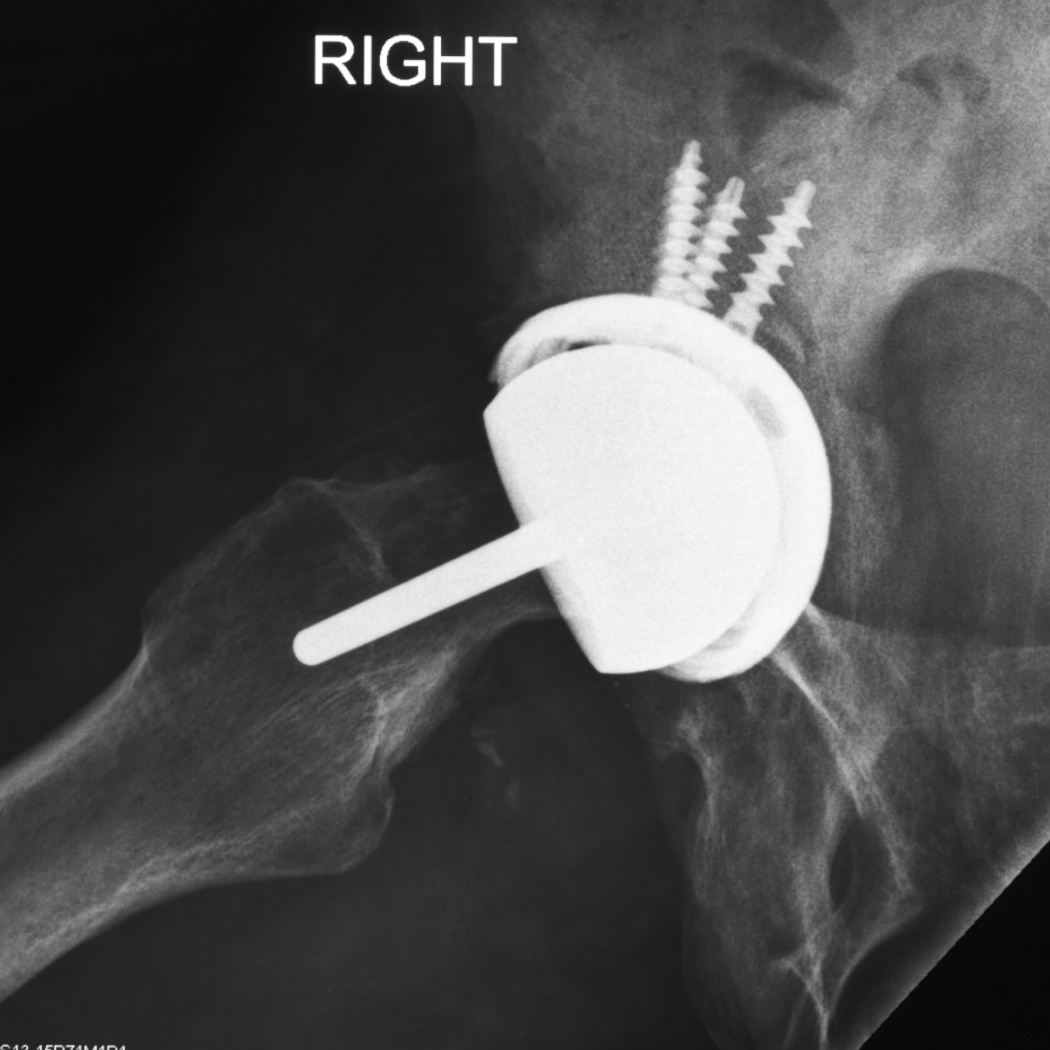
Fig. 9. The lateral radiograph shows the availability of dome screw adjunctive fixation in the compromised acetabular bone.
The rigid monobloc resurfacing acetabular components are difficult to position.2 The radiographic appearance of an acetabular component is a combination of version and abduction. In rough terms each degree of increased anterversion becomes a degree of increase vertical acetabular orientation.
Revision With Polyethylene
Some patients who have developed metallosis are concerned about continuing with a metal-on-metal bearing surface. Women and patients with smaller femoral head sized components present the greatest concerns. If a woman or smaller patient remains an appropriate candidate for resurfacing, revision of the acetabular component to polyethylene is often possible. A standard two-piece, titanium-backed component with a large diameter cross-linked polyethylene inner bearing is used (Fig 8, 9, and 10). Alternatively, using a cemented all-polyethylene acetabular prosthesis with or without a titanium acetabular cage is possible. Placement of these components is similar to conventional total hip replacement.

Fig. 10. This photograph shows a currently available resurfacing prosthesis with a titanium backed polyethylene acetabular bearing surface as an alternative to metal-on-metal.
The results of these procedures are favorable for most patients and recovery is quicker compared to both the initial resurfacing procedure and revision to total hip replacement. Concerns remain about the long-term durability of polyethylene particularly in the larger and thinner sizes.26-28,45 Cemented acetabular components may loosen over time but the longevity with this solution is often many years.26-28
When presented the option of polyethylene resurfacing patients often question the durability of this material. The wear simulator data available at this time suggest, that even with the larger diameter femoral components, ten of more years of useful life can be expected. Most resurfacing patients are young and they will need more than ten years use from their prosthesis. If wear through occurs, a straightforward revision to another polyethylene liner is possible. Also, surveys have consistently shown that given the tradeoff between prosthesis survivorship and function patients choose function. Therefore, polyethylene resurfacing remains a reasonable option for many patients.
It is possible to perform hip resurfacing surgery using a nitrated titanium femoral prosthesis with a polyethylene acetabular component but published long term data with this option are not readily available. From 1995 until 2005 we used a ceramic femoral prosthesis with a cemented polyethylene or two piece acetabulum with satisfactory outcomes. The FDA reclassified the femoral device and additional testing will be required for continued use.28,29
Polyether-ether-ketone (PEEK) is widely used in orthopedic applications and has been used as a bearing surface for joint replacement.24 We have used PEEK for the acetabular component and it remains under development in Italy and is not available at this time.
Revision to Total Hip Replacement
Converting the resurfacing prosthesis to a total hip replacement is always available. It is possible to do this by retaining the metal acetabular component and using a large-diameter, ceramic femoral prosthesis. Long-term information on this option is not yet available but ceramic-on-metal articulations have shown favorable wear characteristics.44
Usually, conversion to total hip replacement is performed by placing a two-piece, metal-backed, high-density polyethylene acetabular component and a stemmed femur. The metal customarily chosen for both components is titanium. It is important, for this option, that the surgeon has substantial experience with revising resurfacing prostheses. The acetabular component of the resurfacing prosthesis can be removed easily, but the femur rarely is loose and it must be removed very carefully to preserve the surrounding bone in the femoral neck. Broaching the femur can be very challenging. The former channel for the stem of the resurfacing component must be appreciated and bypassed carefully.
Instability is a notable concern in performing a revision from a resurfacing prosthesis to a total hip prosthesis. The reasons for these concerns are multifactorial. The revised hip will have a smaller femoral head diameter than the resurfacing. Also, there has been a more extensive capsulectomy performed as part of the resurfacing.14 In addition, the patient has been accustomed to the greater security of resurfacing and may remain highly active. Using a relatively large ball diameter and very careful technique is recommended. Often a femoral head diameter of 40 mm or in some instances 44 mm is possible. Close follow-up of patients is recommended if only smaller diameter components are used. To avoid further compromise to the soft tissues, the same surgical approach as used for the resurfacing procedure is recommended.
Results
We have evaluated 311 patients with concerns of noise, pain, swelling, and/or instability following hip resurfacing; 137 were found to have metallosis and the remainder a variety of other diagnoses. Of the 137 patients diagnosed with metallosis, 122 have undergone revision surgery. There have been 71 resurfacing revisions. 69 patients have a successful outcome with up to 10 years of follow-up). Fifty-one patients underwent revision to a total hip replacement and 46 have a successful outcome. Two patients have had a second revision, one a third revision, and two patients retain their replacement but do not consider it successful. Only one patient surgically treated continues to show signs of metallosis. There have been no instances of systemic cobalt toxicity.
References
- 1. Bin Nasser A, Beaulé P, O’Neill M, Kim R, Fazekas A. Incidence of groin pain after metal-on-metal hip resurfacing. Clin Orthop Relat Res 2010; 468:392-399.
- 2. Browne J, Bechtold C, Berry D, Hanssen A, Lewellen D. Failed metal-on-metal hip arthroplasties. Clin Orthop Relat Res 2010;468:2313-2320.
- 3. Bohlman HR. Replacement reconstruction of the hip. Am J Surg. 1952: 84(3): 268-278.
- 4. Charnley J. Arthroplasty of the hip. A new operation. Lancet. 1961;1:1129-1132.
- 5. Coleman RF, Herrington, J, Scales JT. Concentration of wear products in hair, blood, and urine after hip replacement. Br Med J. 1973;1:527-529.
- 6. De Haan R, Campbell PA. Su EP, De Smet KA. Revision of metal-on-metal resurfacing arthroplasty of the hip: the influence of malpositioning of the components. J Bone Joint Surg Br. 2008; 90:1158-1163.
- 7. Della Valle CJ, Nunley RM, Raterman SJ, Barrack RL. Initial American experience with hip resurfacing following FDA approval. Clin Orthop Relat Res 2009:467:72-78.
- 8. Ferguson AB Jr, Laing, PG, Hodge ES. The ionization of metal implants in living tissues. J Bone Joint Surg Am. 1960; 42:77-90.
- 9. Giménez Camarasa JM. Cobalt contact dermatitis. Acta Derm Venereol 1967;47(5):287-292.
- 10. Haboush EJ. A new operation for arthroplasty of the hip based on biomechanics, photoelasticity, fast-setting dental acrylic, and other considerations. Bull Hosp Joint Dis. 1953;14:242-277.
- 11. Hallab NJ, Anderson S, Caicedo M, Skipor A, Campbell P, Jacobs JJ. Immune responses correlate with serum-metal in metal-on-metal hip arthroplasty. J Arthroplasty 2004;19(8 Suppl 3):88-93.
- 12. Hing C, Back D, Baily M, Young D, Dalziel R, Shimmin A. The results of primary Birmingham hip resurfacings at a mean of five years: an independent prospective review of the first 230 hips. J Bone Joint Surg Br. 2007;89:1431-1438.
- 13. Jones DA, Lucas HK, O’Driscoll M, Price CH, Wibberley B. Cobalt toxicity after McKee hip arthroplasty. J Bone Joint Surg Br. 1975; 57: 289-296.
- 14. Lachiewicz PF. Metal-on-metal hip resurfacing -- a skeptic’s view. Clin Orthop Relat Res. 2007; 465:86-91.
- 15. Langton DJ, Jameson SS, Joyce TJ, Webb J, Nargol AV. The effect of component size and orientation on the concentrations of metal ions after resurfacing arthroplasty of the hip. J Bone Joint Surg Br. 2008;90:1143-1151.
- 16. Lewinnek GE, Lewis JL Tarr R, Compare CL,, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978 60:217-220.
- 18. McBride ED. A metallic femoral head prosthesis for the hip joint. J Int Coll Surg. 1951;15(4):498-503.
- 19. McKee G. McKee-Farrar total prosthetic replacement of the hip. In: Jayson M, ed. Total Hip Replacement. London: Sector Publishing; 1971:47-67.
- 20. McKee GK, Watson-Farrar J. Replacement of arthritic hips by the McKee-Farrar prosthesis. J Bone Joint Surg Br.1966; 48:245-259.
- 21. Moore AT, Bohlman HR. Metal hip joint: a case report. J Bone Joint Surg Am. 1943;25:688-691.
- 22. Müller ME. The benefits of metal-on-metal total hip replacements. Clin Orthop Relat Res. 1995;311:54-59
- 23. Nunley RM, Zhu J, Brooks PJ, Engh CA Jr, Raterman SJ, Rogerson JS, Barrack RL. The learning curve for adopting hip resurfacing among hip specialists. Clin Orthop Relat Res; 2010; 468:382-391.
- 24. Pace N, Marinellia M, Spurio S. Technical and histological analysis of retrieved carbon fiber-reinforced poly-ether-ether-ketone composite alumina bearing liner 28 months after implantation. J. Arthroplasty 2008;23:151-155.
- 25. Pandit H, Glyn-Jones S, McLardy-Smith P, Gundle R, Whitwell D, Gibbons CL, Ostlere S, Athanasou N, Gill HS, Murray DW. Pseduotumours associated with metal-on-metal resurfacings. J Bone Joint Surg Br. 2008;90:847-851.
- 26. Pritchett J. Curved-stem hip resurfacing: minimum 20-year followup. Clin Orthop Relat Res. 2008;466:1177-1185.
- 27. Pritchett J. Success rates of the TARA hip. Am J Orthop. 1998;27:658.
- 28. Pritchett J. Conservative total articular replacement arthroplasty: minimum 20-year follow-up. In: McMinn DJW, ed. Modern Hip Resurfacing. London: Springer; 2009:408-414.
- 29. Pritchett J. Bearing surface choices and heat generation of hip resurfacing prostheses. eJBJS-Proceedings of the 2009 Annual Meeting of the Association of Bone and Joint Surgeons. accessed September 1, 2010.
- 30. Restrepo C, Parvizi J, Kurtz SM, Sharkey PF, Hozack WJ, Rothman RH. The noisy ceramic hip: is component malpositioning the cause? J Arthoplasty 2008; 23:643-649.
- 31. Ring PA. Complete replacement arthroplasty of the hip by the ring prosthesis. J Bone Joint Surg Br. 1968; 50:720-731.
- 32. Schroeder HA, Nason AP, Tipton IH. Essential trace elements in man: cobalt. J Chronic Dis 1967;20(11):869-890.
- 33. Smith-Peterson MN. Arthroplasty of the hip. A new method. J Bone Joint Surg Am. 1939;21:269-288.
- 34. Sullivan J, George R, Bluvas R, Egan J. Myocardiopathy in beer drinkers: subsequent course. Ann Int Med. 1969;70:277-282.
- 35. Thompson FR. An essay on the development of arthroplasty of the hip. Clin Orthop Relat Res. 1966;44:73-82.
- 36. Townley CO, Walker S. Intramedullary cup-stem arthroplasty of the hip J Bone Joint Surg Am. 1961; 43:602.
- 37. Urist MR. The principles of hip-socket arthroplasty. J Bone Joint Surg Am. 1957; 39(4):786-810.
- 38. Venable CS, Stuck WG. Electrolysis controlling factor in use of metals in treating fractures. JAMA 1938;111:1349-1352.
- 39. Walker PS. Human joints and their artificial replacements. 1977 383-393 Springfield, IL: Charles C. Thomas.
- 40. Walker PS, Erkman MJ. Metal-on-metal lubrication in artificial human joints. Wear 1972; 21:377-391.
- 41. Walker PS, Gold BL. The tribology (friction lubrication and wear) of all-metal artificial hip joints. Wear 1971;17:285-299.
- 42. Walker PS, Salvati E, Hotzler RK. The wear on removed McKee-Farrar total hip prostheses. J Bone Joint Surg Am. 1974;56:92-100
- 43. Walter WL, Waters TS, Gillies M, Donohoo S, Kurtz SM, Ranawat AS, Hozack WJ, Tuke MA. Squeaking hips. J Bone Joint Surg Am. 2008; 90 (Suppl 4):102-111.
- 44. Williams S, Schepers S, Isaac G, Hardaker C, Ingham E, van der Jagt D, Beckron A, Fisher J. The 2007 Otto Aufranc Award. Ceramic-on-metal arthroplasties: a comparative in vitro and in vivo study. Clin Orthop Relat Res. 2007:465:23-32.
- 45. Yue EJ, Cabanela ME, Duffy GP, Heckman MG, O’Connor MI. Hip resurfacing arthroplasty: risk factors for failure over 25 years. Clin Orthop Relat Res. 2009;467:1528-1132.
