Ceramic BMHR
Ceramic Birmingham Mid-Head Resection
Info:
 The Ceramic-on-Metal BMHR is an alternative bone-conserving hip implant for patients with metal allergies.
The Ceramic-on-Metal BMHR is an alternative bone-conserving hip implant for patients with metal allergies.
The BHR and BMHR are Metal-on-Metal (MoM) hip joints which produce small amounts of metal wear over the course of time. This metal wear can sometimes cause adverse reactions in patients with metal allergies. A very small percentage of patients (0.3%) from Mr McMinn's BHR series have had adverse tissue reactions requiring revision surgery - all of these patients were women. However, we have never knowingly carried out a MoM joint in any patient with a history of metal allergies.
We now perform a straightforward blood test for any patient who has a suspected metal allergy during their consultation. The blood test is called a Lymphocyte Trasformation Test (LTT) and results are usually available in 2-3 weeks. Mr McMinn is getting increasing numbers of patients who wish to have a conservative hip implant but have a metal allergy (indicated by an LTT), making them unsuitable for a MoM arthroplasty.
Mr McMinn is now able to offer an alternative bearing for patients with a metal allergy, called the Ceramic-on-Metal BMHR. Early results with this type of device show significantly lower metal wear than with any Metal-on-Metal hip replacement - see 'Results' in the next tab for more information. Our numbers and long-term experience with the Ceramic BMHR are limited but in the absence of a better alternative this is currently our best option for patients with a known metal allergy and who wish to have a conservative arthroplasty.
The BMHR (Birmingham Mid Head Resection) stem was originally developed in 2003 for use in patients with poor quality bone in the femoral head. These patients with severe cystic change in the femoral head or with osteonecrosis (dead bone in the femoral head) are not ideal candidates for hip resurfacing. In such patients the BMHR has been giving excellent results because it allows the poor quality bone to be removed, with the stem implanted securely within good bone.
bone in the femoral head. These patients with severe cystic change in the femoral head or with osteonecrosis (dead bone in the femoral head) are not ideal candidates for hip resurfacing. In such patients the BMHR has been giving excellent results because it allows the poor quality bone to be removed, with the stem implanted securely within good bone.
With a regular BMHR, the stem is fitted with a metal femoral head component. For patients with a metal allergy, the stem is fitted with a custom-made ceramic head, specifically manufactured for the individual patient’s anatomy. The ceramic head articulates within a metal cup - the same cup used with for a BHR - so we call it a Ceramic-on-Metal (CoM) joint. The materials and the design of the ceramic head are optimized in order to generate very low wear. And these low wear results cannot be applied to other CoM devices on the market.
Generally speaking the CoM BMHR provides all the benefits of a conservative MoM hip arthroplasty - a good range of movement, excellent stability, reduced risk of dislocation and better revision options - for patients with a metal allergy who wish to lead an active lifestyle after surgery.
Nineteen patients have so far received the CoM BMHR and in every case it was because the patient was not suitable for a Metal-on-Metal hip implant.
Results:
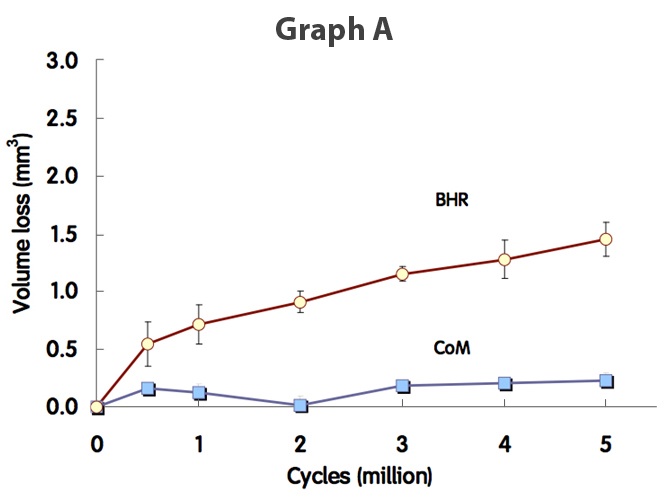
Extensive hip simulator testing has been carried out on the Ceramic BMHR by Smith & Nephew Orthopaedics. Graph A (below) shows the metal wear produced by a regular BHR (red line) and the metal wear produced by a CoM BMHR on a hip simulator. The tests indicate that the wear produced by the CoM BMHR is phenomenally lower than the wear from a regular BHR.
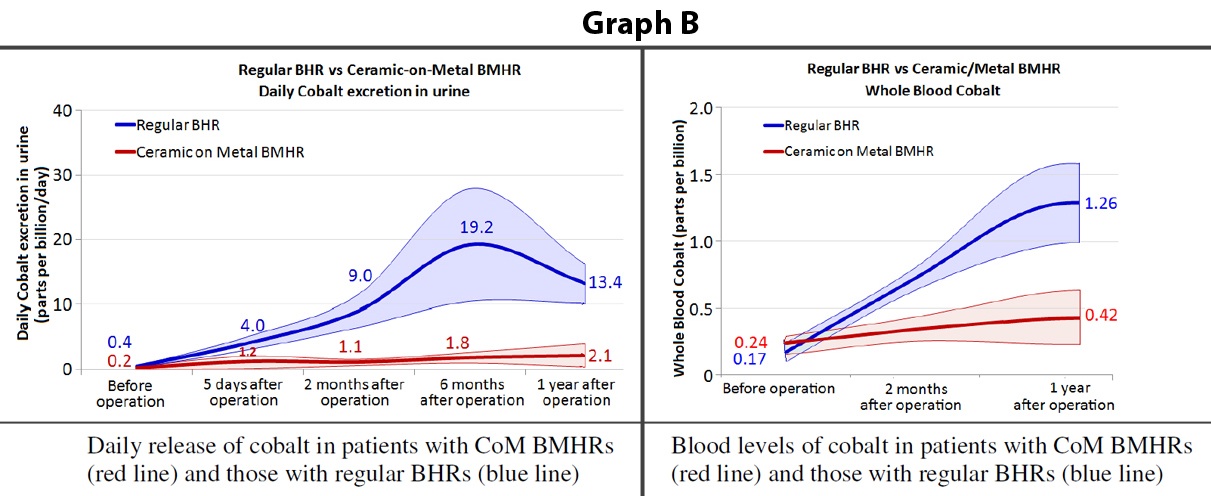
We have also followed up our patients fitted with a CoM BMHR with frequent blood and urine tests. These tests give a good indication of the amount of metal wear produced by the implants over the course of time. Graph B (below) shows the metal ion release (in both blood and urine) in patients with a regular BHR (blue line) and in patients with a CoM BMHR (red line). The information from these results confirm the hip simulator findings - that the metal wear levels in patients with a CoM BMHR are considerably lower than the levels seen in patients with BHRs.
Thanks to the McMinn Centre for providing this information, for more info click here
